In a small study, a bivalent mRNA vaccine targeting omicron BA.4-BA.5 sublineages resulted in similar neutralization of other SARS-CoV-2 variants as a fourth dose of a monovalent mRNA vaccine.. Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for a 10-µg booster dose of their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine in children 5 through 11 years of age. Pending recommendation from the Centers for Disease Control and Prevention (CDC), 10-µg doses will be.

FDA authorizes bivalent COVID19 boosters for children ages 5 to 11

COVID bivalent booster dose Moderna or Pfizer? When should I get it?

Pfizer COVID vaccine vials may hold extra doses, adding to US supply

As Moderna looks to increase the doses in vaccine vials, the White House announces an expected
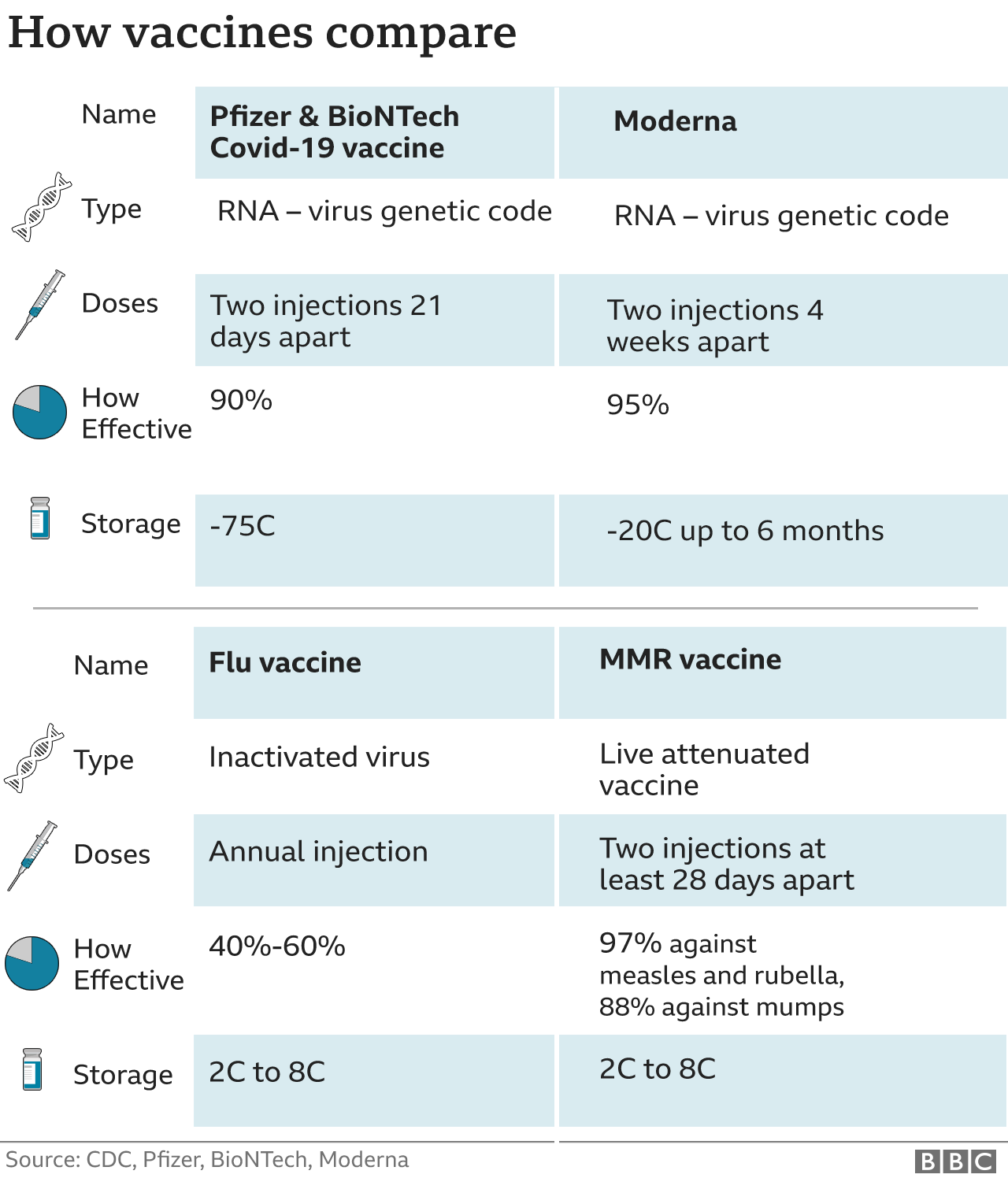
Moderna vaccine safe and effective, say US experts BBC News
FDA emite uso de emergencia para la vacuna contra covid19 de Pfizer
Latest COVID Bivalent Booster Now Available at QNHCH The Queen′s Health System

Pfizer and Others Are Planning for Covid Vaccine Boosters The New York Times
Pfizer to begin vaccine production in Dublin BBC News
Covid19 Patients urged to get vaccine as soon as they can BBC News

COVID Pfizer, Moderna omicron bivalent boosters confuse providers

Pfizer fined 106M for 2600 price hike on epilepsy drug

Does the Bivalent Booster Entirely Replace Other Covid19 Boosters? The New York Times
Pfizer seeks FDA greenlight for bivalent COVID dose in kids under 5 years Ars Technica
Pfizer’s Covid19 vaccine study excluded people with a history of severe allergic reactions
Vaccine Ready

US authorises the use of dualvariant covid19 vaccine boosters New Scientist
Asesores de la FDA autorizar vacuna contra covid19 de Pfizer para niños entre 5 y

Health Canada OKs Pfizer’s bivalent Omicron BA.4/BA.5 booster for kids 511 National
Covid vaccine Pfizer says ’94 effective in over65s’ BBC News
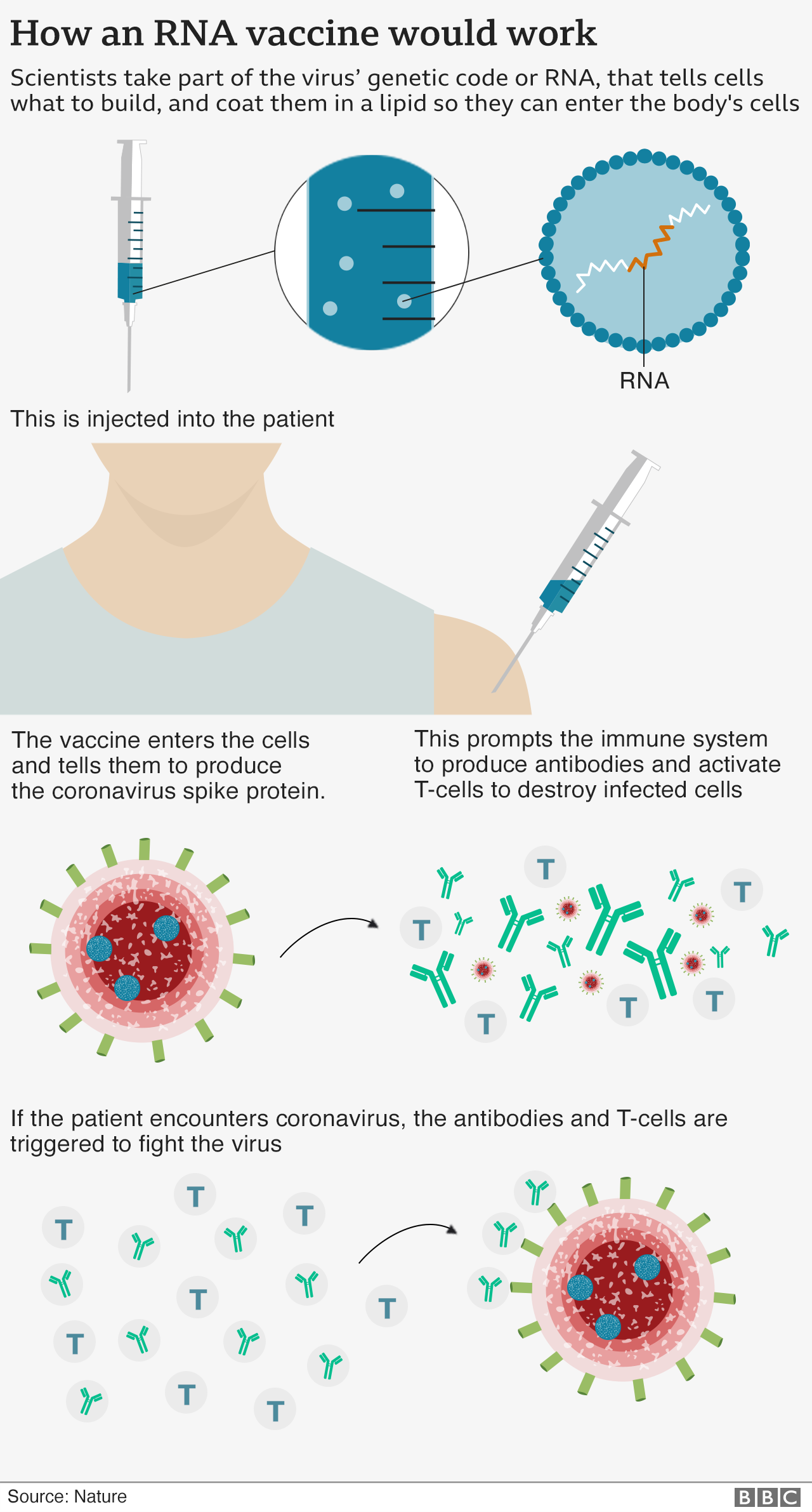
Pfizer’s new BA.4/5 bivalent vaccine differs from its previous formulation by replacing mRNA of the BA.1 Omicron subvariant with mRNA that encodes the BA.4/5 spike protein instead.. However, the eligibility criteria shared by the Department suggest that individuals aged 18 and over can choose either of the four bivalents: Pfizer BA.1, Moderna BA.1, Pfizer BA.4/5, and Moderna.